According to statistics from the National Health and Family Planning Commission, the current incidence of lung cancer in my country is increasing at an annual rate of 26.9%. In recent decades, the number of lung cancer patients has doubled every 10 to 15 years. The results of the third survey of causes of death among residents in my country also show that lung cancer mortality has increased by 465% in the past 30 years, replacing liver cancer as the malignant tumor with the highest mortality rate in China.
Facing such a rapidly growing group of lung cancer patients, I have a deep understanding! In late September 2013, my mother called me suddenly and told me that my father had been diagnosed with non-small cell lung adenocarcinoma the day before. It was like a thunderbolt in the clear sky, and I couldn't believe it was true: "God! Why did my dearest dad have a terminal illness!"
Taking advantage of the National Day holiday, I hurried back home. What makes me gratified is that my father is still so tough and looks good. It's just that his voice is no longer able to speak, and his expression is obviously more depressed. I kept encouraging my father, but my father just smiled most of the time. In fact, he should know a lot of things better than us. Of course, hope remains.
On October 8, his father, accompanied by his family, went to the hospital. Surgery was soon performed, and then chemotherapy was performed. After the first course of chemotherapy was over, considering the inconvenience of taking care of his father, he adopted the method chosen by most families: returning to the county where he was home to continue the treatment.
Later, I heard a doctor's introduction that I also bought small-molecule targeted drugs for my father. The price of the drugs put me under pressure. But for the sake of my father, I must persist in suffering! When I saw my father again, I felt extremely uncomfortable: all the black hair on my father's head had fallen off, and he was weak and looked at least ten years old. The father who used to be full of spirits will never be seen again.
In April 2014, his father was reviewed at the hospital. The results of CT examination showed that no obvious tumors were found in both lungs of the father. Blood tests are also normal. As soon as the news came out, our family was very happy and felt that all the efforts were worthwhile. But this joyful time is always too short. In February 2015, my mother called and told me that due to my father’s recent cough and chest tightness, he went to the hospital for an examination and found that the lung cancer had recurred and brain metastases had occurred. "At this time, I remembered that a doctor friend said to me that chemotherapy will significantly reduce lung cancer lesions, or even disappear, but relapse quickly. Although small-molecule targeted drugs are effective, they usually appear resistant within 9-12 months. The medicine fails.

My father's condition relapsed. Although I was very sad, I did not give up the hope of finding an effective treatment for my father. Later, I went to a well-known cancer specialist hospital to consult a doctor. The doctor carefully reviewed my father’s medical records and suggested that my father received ACTL targeted cellular immunotherapy. The doctor told me that surgery, chemotherapy, radiotherapy and biological therapy are the four major treatment methods of contemporary anti-tumor therapy. Cellular immunotherapy is one of the most important components of biological therapy. ACTL treatment is a typical precision medical technology, which is safe and reliable, without toxic side effects, and can accurately identify and kill cancer cells, but has no killing effect on normal tissue cells. It can also delay or even prevent the occurrence of drug resistance of small molecule targeted drugs for the treatment of lung cancer, and significantly improve the quality of life. After listening to the doctor's introduction, I felt as if I had caught a life-saving straw, and in despair I saw possible hope.
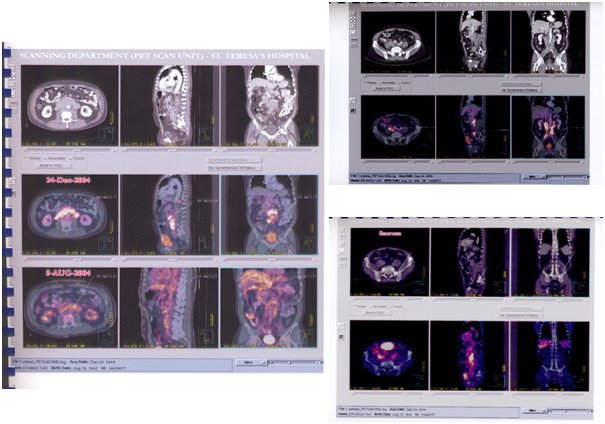
In response to my father's condition, the doctor formulated an ACTL targeted cellular immunotherapy program for him twice a month. After 6 treatments, serum tumor markers returned to normal. Imaging report: no new lesions were found, and brain metastases were significantly reduced. The following is a picture of the situation before and after treatment:
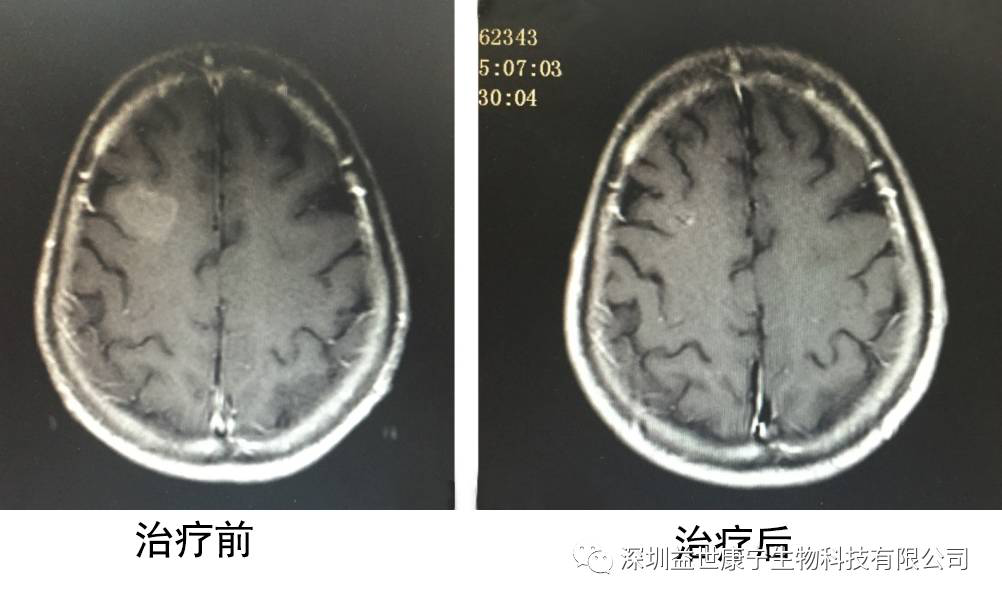
Seeing my father miraculously improved again, I was ecstatic. After ACTL targeted cellular immunotherapy, his father, who was declared to be only alive for more than three months at the time, is now more and more healthy after two years, and he is gradually regaining his vigor. And I have a very good appetite and I have gained weight. Here, I want to thank the doctor and all those who helped us. Thank you!
Lung cancer is one of the malignant tumors with the fastest increase in morbidity and mortality and the greatest threat to the health and life of the population. In the past 50 years, many countries have reported that the incidence and mortality of lung cancer have increased significantly. The incidence and mortality of lung cancer in men are the first of all malignant tumors, the incidence of women is the second, and the mortality is the second.
Traditional treatment options for lung cancer include surgery, radiotherapy and chemotherapy. However, surgery cannot remove metastatic lesions and residual tumor cells, and the side effects of radiotherapy and chemotherapy significantly reduce the quality of life of patients. In response to these conditions, patients can choose ACTL treatment.
【Case Introduction】
Today I am sharing a case of ACTL technology in the treatment of lung cancer brain metastases. Relevant information was provided by the Fifth People's Hospital of Dongguan City, and Shenzhen Yishi Kangning provided technical support for ACTL.
In this case, the patient used ACTL technology alone and after four treatments, the serum tumor marker level was reduced, the brain metastasis lesions were significantly reduced, the condition was effectively controlled, and the mental and physical strength were significantly improved.
【Patient Information】
The patient Zhang, male, 74 years old, was admitted to the hospital for more than half a year after surgery for upper right lung cancer, and dizziness worsened by brain metastasis syndrome after radiotherapy and chemotherapy.
The patient had undergone surgery to remove the tumor in 2014, and received systematic radiotherapy and chemotherapy. In March 2015, he was found to have patchy abnormal signal shadows on the right frontal lobe of the brain and developed dizziness and other uncomfortable symptoms.
【Treatment Information】
In the absence of other treatments, the patient received ACTL immune cell therapy. On June 2, 2015, the peripheral blood of the patient was collected to prepare ACTL immune cells. The patient received the first reinfusion on June 17, and received a total of three reinfusions according to the ACTL treatment plan.
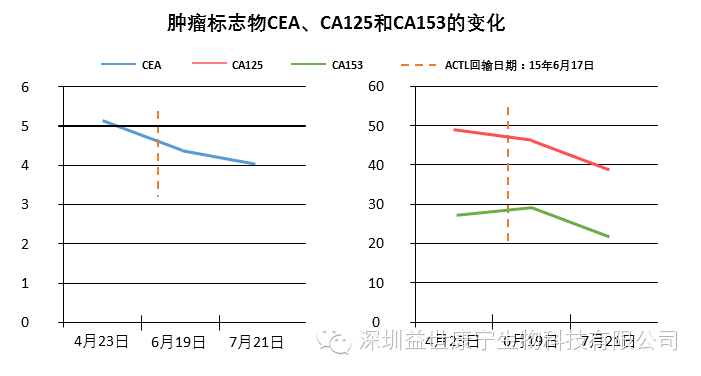
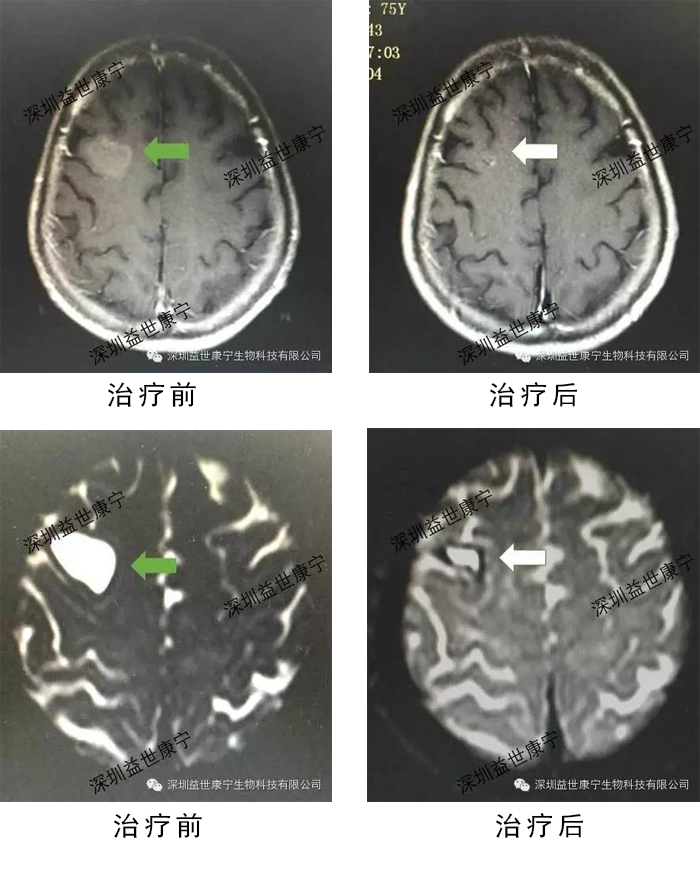
Monitoring the patient's serum tumor marker levels showed that the tumor markers CEA, CA125 and CA153 decreased significantly. Imaging showed that the metastatic lesions in the brain of the patient were significantly reduced.
【in conclusion】
ACTL immune cells are used alone to treat lung cancer brain metastases, and the effect is obvious. The patient's tumor marker levels began to decline during the treatment; after receiving four treatments, imaging revealed that the metastatic lesions in the patient's brain were significantly reduced. The patient's quality of life improved significantly.
The following are the results of tumor marker detection and imaging examination chart.

In 2003, the ACTL technical team received a doctor, but he did not come to see the patient, but to find a new treatment for his disease.
Dr. Zhang was diagnosed with non-small cell lung adenocarcinoma-the most common type of lung cancer. At the time of diagnosis, it was already at an advanced stage and no indication for surgical treatment was lost. At that time, the only clinical option was chemotherapy. Dr. Zhang, who was well versed in chemotherapy, immediately rejected chemotherapy after confirming his diagnosis. After being introduced by a colleague, I got in touch with the ACTL technical team.
According to Dr. Zhang's condition, the ACTL technical team recommended that he receive chemotherapy, combined with ACTL treatment, so that the effect will be better. But Dr. Zhang insisted not to do chemotherapy, only ACTL. The technical team can only respect the opinions of Dr. Zhang himself. The hospital tested him for blood tumor markers and used immunohistochemistry to detect target antigens in tumor tissues.
"Similar to Dr. Zhang's condition, it is usually recommended to use other treatments to reduce the tumor burden as much as possible, and then adopt a comprehensive treatment plan such as ACTL. Using ACTL alone, we were really not sure what the result would be," a member of the ACTL technical team recalled.
The examination results showed that Dr. Zhang’s blood carcinoembryonic antigen (CEA) and cyfra21-1 antigen were positive, and the tumor tissue’s survivin antigen (Survivin), melanoma-associated antigen-A3 (MAGE-A3), and prostate specific membrane antigen (PSMA) were positive Positive. These five antigens are clear targets for ACTL treatment, so Dr. Zhang embarked on his anti-cancer "adventure journey".
Due to the serious illness, the ACTL technical team designed a set of "impact therapy" for Dr. Zhang-three treatments per month, and close clinical observations. Dr. Zhang responded well to the treatment with no obvious side effects. He felt that his mental state had improved significantly, and his years of insomnia had also improved significantly.
After 9 treatments in three months, CT scans found that the lung lesions were reduced by a quarter, and the condition showed a trend of improvement. This completely exceeded the expectations of the technical team.
The ACTL technical team considered it unnecessary to continue to use "impact therapy" and decided to change the treatment plan from the original three treatments per month to twice a month. Doctor Zhang's condition continued to improve.
After two years of treatment, Dr. Zhang's condition improved significantly, and most of the lung lesions disappeared. Below is a comparison chart before and after treatment.
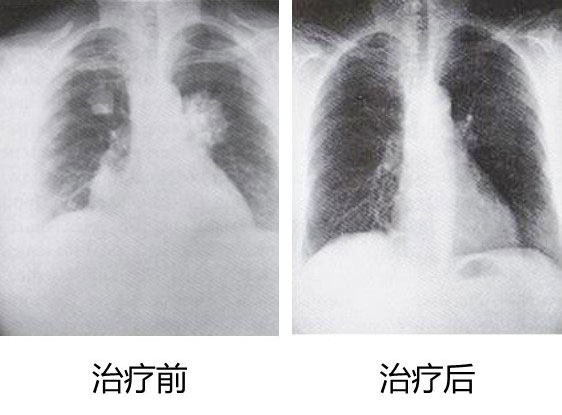
Members of the ACTL technical team were particularly excited when talking about the case: “We never thought that using ACTL alone could make Dr. Zhang’s condition so much improved. The effect is obvious to all, and this case makes us more convinced that we are on the right path. Come on. ACTL can help more patients!"
Dr. Zhang has survived after ACTL treatment. Although the blood CEA level is still high, no signs of recurrence have been found in regular examinations for many years.
The traditional methods of treating lung cancer are mainly through surgery, chemotherapy and radiotherapy, but they often damage normal cells and cause mild or severe side effects. ACTL treatment can accurately identify cancer cells, accurately kill cancer cells remaining in the body after surgery, radiotherapy or chemotherapy, and prevent recurrence and spread; it can also reverse the patient’s resistance to targeted drugs and endocrine drugs, and prolong the patient’s recovery from these types of drugs. Time to benefit from the medicine. The view of modern tumor treatment is individualized comprehensive treatment. Years of clinical practice have shown that ACTL combined with other treatment methods will have better treatment effects.

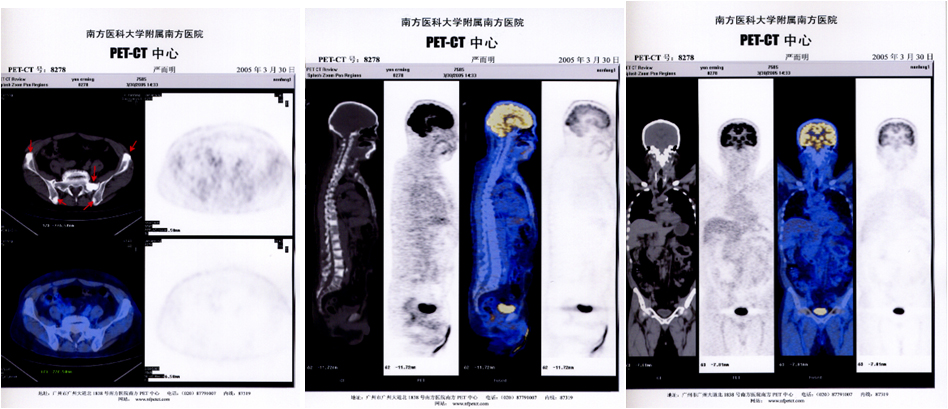
This is a case of stage IV non-small cell lung cancer in a 50-year-old female with lymph node metastasis and cough.
The patient was diagnosed as left lower lung adenocarcinoma by PET-CT, and CEA and other tumor markers were normal. Surgical resection was performed at Guangdong Provincial People's Hospital. Pathological examination: moderately differentiated adenocarcinoma of the left lower lung. Received systematic chemotherapy in the hospital after the operation. After more than 3 years of re-examination of PET-CT, the mediastinal lymph nodes, right upper clavicle, and axillary lymph nodes were diagnosed with swollen lymph nodes. After needle biopsy, they were diagnosed with lung cancer recurrence lymph node metastasis. After six months of oral Iressa treatment, serum CEA continued to rise.
After the patient was treated with CEA/CK19 dual-target ACTL in mid-2012, PET-CT scan showed tumor lymph node metastasis decreased or disappeared, CEA level gradually decreased, and maintained normal level during ACTL treatment, and oral Iressa treatment has been effective.
The following is the change of the patient's tumor marker CEA before and after ACTL treatment.
Summary: The target of ACTL treatment is CEA and CK19. After 6 treatments, tumor markers more than doubled and the lesions disappeared significantly. After 28 months of treatment, the re-examination of tumor markers was normal, and the imaging data showed: no abnormalities were found, and no new lesions appeared. At present, the patient is all normal, working normally, and still adheres to treatment (ACTL treatment + Iressa), and Iressa is still effective.

Cervical cancer is currently one of the few cancers that can be cured. The main cause of cervical cancer is HPV virus infection, among which HPV-16 and -18 are high-risk types. Ms. Zhang found positive for the two types of HPV during the physical examination and was hospitalized for treatment, but the effect was not good. So he started to receive ACTL treatment, and after seven treatments, the examination results showed that "no papilloma virus infection was seen."
The patient Zhang, female, was tested positive for HPV16 and HPV18 in his cervical HPV (papillomavirus) in January 2015. In the absence of other treatment, the patient received ACTL treatment. After seven treatments, the patient was re-examined in mid-November 2015, and the result indicated that "no papillomavirus infection was seen."
Patient information: female patient, 35 years old
Diagnosis: (1) Cervical intraepithelial neoplasia grade II (2) Cervical HPV infection
Treatment history: On April 18, 2015, under the basic anesthesia, the cervix ring electrotomy was performed.
On August 11, 2015, ACTL treatment was performed for HPV targets.
ACTL treatment plan: treatment twice a month, treatment time 3 months. No other treatments were combined during the entire treatment period.
ACTL treatment result: HPV turned negative. During the treatment, there was no toxic side effect.


Compared to the pain, what is more painful than the pain is to uncover my scars again. I have experienced a terrible disaster. It has been more than 6 years since this disaster.
Six years ago, when I was doing housework, I suddenly felt pain in my lower abdomen, and some brown secretions flowed out of my lower body intermittently. At that time, I didn’t care too much because of inflammation. These symptoms continued for more than half a month and still did not relieve. My husband and I Only realized the seriousness of the condition. So I went to the hospital for an examination and was diagnosed with cervical cancer. When I just learned the news, I felt like a bolt from the blue.

I know that I may not live long, and I often cry. There was only one question in my mind all day, "What should I do now?" I had two children to raise. At that time, one child was in college and the other was in high school. I couldn't leave them behind. I was more than a patient. , Is a mother. I think every mother is great, maybe it is this belief that inspired my strong desire to survive.
So I embarked on an extremely difficult road to survival. On the recommendation of the doctor, I received radiotherapy first and then chemotherapy. Thinking back to that period of treatment, life is better than death.
Although the process was painful, during the days I was receiving treatment, I lived from one week to the next, from this physical examination to the next. Relatives and friends often visit me and enlighten me. My family cares about me very much, and they give me hope. When I was in pain, I thought that I must survive this week, to the next chemotherapy, and to the next month, I want to survive. This is how I set goals for myself, and the realization of the goals helped me through the dark night after another.

At that time, I often prayed that this kind of treatment would buy me more time, after all, life and death are only in the middle of the day. I just didn't expect that the thing that worries me the most is coming. Cervical cancer recurred, and obvious metastatic lesions appeared in the pelvic cavity. At that time, after the attending doctor saw the results of the PET-CT examination, he told me that I had to change the treatment method, otherwise the condition might get worse. After consultation with hospital experts, I was advised to accept ACTL anti-tumor targeted cellular immunotherapy. What struck me the most was that my husband agreed without even thinking about it. He told me in the future: "As long as there is a glimmer of hope, never give up!" After hearing this, he held him and cried for a long time, releasing the long-repressed emotions. Because my cervical cancer is caused by human papillomavirus type 16 (HPV-16) infection, ACTL targets HPV-16 antigens and tumor antigens. After 6 months of ACTL treatment, twice a month, After half a year, my metastatic disease basically disappeared. This is the best news that our family has heard in more than two years. A few months passed and the tumor did not recur. One year, two years, three years, and four years passed, and time passed, and the tumor did not recur. I am very grateful to all the people who helped me, especially the doctor in charge, without him helping me to develop the correct treatment, how could I have a second life. How could they have witnessed two children graduated from college to start a family.
In fact, during the treatment period, besides being unwilling to give up the hope of life, the most important thing is that I still insist on running, exercising, chatting with my family every day, eating only a healthy diet, having a regular schedule of work and rest, going to bed early and getting up earlier than normal. Life is better. Although I defeated the disease, the mention of this incident still leaves me lingering. The tumor didn't beat me, but I still had sequelae. But I am still grateful for life, because I survived, I am grateful that I have everything I have.
I understand the psychology of women who just learned of cancer. Understand their panic, worry, and frustration, because I have experienced them all. It is easy to be depressed and fall into pain, but you must let go of these emotions, otherwise they will slowly erode you, because fear is more painful than illness. Life is simple, don't make it complicated. After the pain and anxiety are properly released, fight the illness in the correct way. After the long night, the sunrise at dawn will be more beautiful.

Cancer, are you scared when you hear these two words? I don’t know if you are afraid or not. Anyway, seeing these two words in my medical history, I am really afraid, I am afraid, I am confused, I am at a loss!
Allow me to share with you all the nightmares and beauty that I have experienced over the past year.
The nightmare is coming, you hold my hand
The nightmare started in December of the previous year. I was enjoying a comfortable retirement life, but my buttocks were unknowingly painful, especially when I was moving, and my right leg gradually felt weak. I didn’t think it was because of the weather. It is caused by factors such as, diet, activity, etc., and the symptoms appear repeatedly. Half a year later, my family found that something was wrong with me going on like this, so my lover accompanied me to the hospital and did a lot of inspections. At the end of the inspection, my heart was disturbed. A woman’s sixth sense told me that this time it might be possible. Something bad happened.
After all the examination results came out, the doctor and my lover said something quietly outside the office, and then walked in very calmly. My lover also quietly walked to me, holding my hands, I felt cold The hands were still trembling a little, and the doctor said: "Ms. H, hello, I have communicated with your lover just now. He wants me to tell you your condition completely. I hope you can face everything in a strong and correct way. The result of the examination was multiple osteolytic destruction throughout the body, considering the possibility of multiple myeloma..."
what! At that time, I felt that my sky was about to fall. I didn't say a word. There were only three words "myeloma" in my mind...

I woke up from the dream
As soon as the news that I had cancer spread, family, relatives and friends came to greet me, sent me positive energy, and tried every means to inquire about the high-end technology in the oncology field for me, and visit oncologists everywhere for me. I know that my relatives and friends dare not say anything in front of me, fearing that I will be sad when I hear them, and they will stop talking, but they will not know where to start.
Later, a series of examinations were performed, and in June 2016, it was finally diagnosed as occult breast cancer with bone metastases throughout the body. In other words, no cancer was found in the breast, but the breast cancer cells have spread to the bones of the whole body.
I am afraid, so afraid that I dare not look into the eyes of my relatives, because I can feel your deep love and sadness for me! I am sad, and the sentimentality of a woman makes me shed painful tears. When I am alone, I feel that my breathing is painful! I was terrified, looking forward to seeing the results of the next physical examination, and waiting, it was the most painful torture!
See the truth in suffering! Seeing all the people around me running around for me day and night, trying to find the light of life for me, even if there is only a glimmer of hope. The most romantic thing I can think of is getting old with you, with you, with you! No matter how cold my heart is, you will come to warm me up. I am in pain, but I am happy!
With you, love me so much, I must face reality firmly, I can't be overwhelmed by that nightmare, I will use my best to fight cancer, no longer let tears stay with me overnight.
The dawn is here, you help me continue to write a better life
Hard work pays off! In order to reduce my pain, my family has been working hard to find a better treatment method for me. Fate made me encounter ACTL technology.
My family also gave me a professional class, and now I simply share it with you-ACTL is called ACTL Targeted Anti-tumor Cell Immunity Technology. It was developed at the University of Arkansas in the United States and is in collaboration with the United States, Germany, Italy, Japan, Taiwan has carried out a number of scientific research cooperation, has obtained patent authorization in China, the United States, the European Union and other countries, and obtained more than 20 international and domestic new patents within 5 years. My family told me many successful examples, and I was full of joy. It made me feel that ACTL was the dawn of my gloomy days and brought me more hope. Soon, with the help of my family, I checked relevant indicators. For the treatment, I finally chose the treatment plan of endocrine therapy combined with ACTL.
Traditional treatment methods usually require regular hospitalization. ACTL treatment does not require hospitalization. I really like this. I can still live at home normally. I only need to do blood draw and cell infusion twice a month, every half an hour. About, compared with chemotherapy and endocrine therapy, ACTL treatment has almost no adverse reactions. After the combined treatment, my body is much better than before, my hip is no longer painful, and my right leg is no longer weak. The tumor markers examined in the hospital have declined, and the imaging results also show that my condition has improved. All these results are better than I expected. My relatives and friends are very excited when they see my current state of mind. They didn't expect me to heal so quickly, looking like a healthy person. If I simply use traditional treatment methods, I'm really worried that my body can't bear it, and I can't imagine what life will be like that.
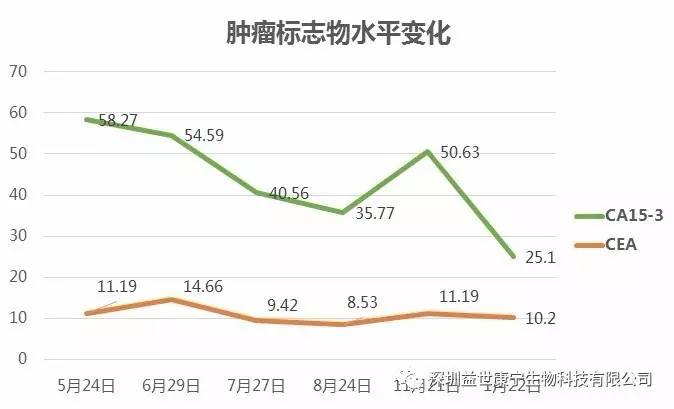
The patient's tumor marker levels continued to decline after starting ACTL treatment
I can have today. I want to thank the family who brought ACTL to me and the ISKCON team. It is you and you, who brought me the dawn of life and helped me continue to write a beautiful life.
I hope to use my modest strength to tell the people around me about this great technology. I hope they can be as lucky as me and let us fight cancer together!

Breast cancer is the number one common malignant tumor in women. The American Cancer Society pointed out that approximately 130 million women are diagnosed with breast cancer each year, and approximately 465,000 women die of breast cancer. The incidence of breast cancer is on the rise globally, with an annual increase of 2%-3%. , Developing countries are particularly rapid. Compared with other cancers, breast cancer patients suffer not only physical pain (the lack of female secondary characteristics), but also psychological pain (loss of self-confidence) after surgery.
With the continuous increase in the incidence of breast cancer in my country, the younger generation of the disease, and the gradual development of early diagnosis technology, breast-conserving surgery has gradually replaced modified radical mastectomy for breast cancer, becoming the preferred surgical method, and combined with biological treatment, the treatment of breast cancer More targeted, more scientific and more rational, the era of "private customization" has arrived.
Case of ACTL technology for breast cancer treatment
This is a comparison before and after targeted T cell therapy for a breast cancer patient with advanced bone metastasis. Before receiving treatment, there were multiple metastases in the spine and severe pain.
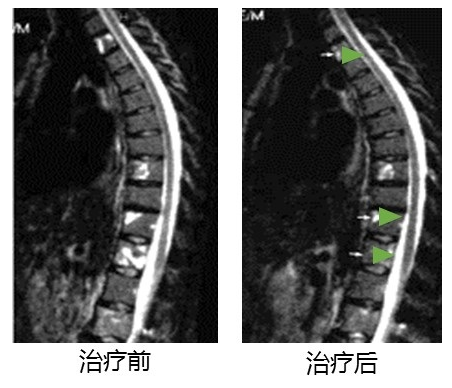
According to immunohistochemical tests, the patient’s tumor cells express Her2/neu, CEA and BA46 tumor-associated antigens, which are the attack targets of ACTL. The infused T cells can accurately eliminate tumor cells expressing these antigens. After four months of treatment, spinal metastases were significantly reduced, the patient's pain was reduced, the condition was significantly improved, and the quality of life was significantly improved. The patient has survived for more than 6 years.

"I really envy your current state, as indifferently as a breeze, and solid as a rock in your heart."
Last night, while chatting with young breast cancer girl Li Wei (pseudonym) on WeChat, she suddenly said this to me. During the chat, I could deeply feel the intense anxiety in her heart, and the tangled and helpless words swept through me like a machine gun.
She must be very helpless, entangled, and low now, but I can't do anything. I can only accompany her silently and comfort her: "Since you can't change what has happened, be prepared to adapt to everything." To encourage her by telling her about another friend suffering from breast cancer.

On March 23, 2011, my friend found a lumps on the right breast for the first time. I went to the hospital for an examination. The doctor said that there was nothing serious but breast hyperplasia. Don't worry, she was relieved after hearing the doctor's words.
At that time, my friend had just graduated and was very busy at work. Even fever did not stop her from working. Because I am afraid of stopping, all efforts will be lost. In addition to being busy or busy, there is no time to take care of the "small lumps" that appear in the breasts. I think that since the doctor said that there is no problem, there will be no big problems. Who would have expected that this lumps would eventually drive her to breast cancer Where is the abyss?
On June 12, 2011, the pain became unbearable because of the enlarged lumps in the breast. She went to the hospital for an examination again, but this time she was told that she had breast cancer. After that, she was preparing for surgery. She couldn't accept the physical disability, especially for the most important part of women-the missing breast. She felt that there was no difference between living and dying without a breast. When she was still in the sadness of missing breasts, another news hit her hard again-breast cancer has returned! She once thought of passing away like this and dying!

Seeing that she has no love for life, family and friends took turns to accompany her and encourage her, she regained her confidence and learned about ACTL's targeted anti-tumor cell immune technology with the help of friends. At that time, because she was a breast cancer patient with advanced bone metastasis, her spinal metastasis was severe and she had severe pain. In response to this condition, the professor of Ishikoning used ACTL with CEA, BA46 and Her-2/neu antigens for 4 months. After 4 months, her spinal metastases were significantly reduced and the pain was relieved. She has survived after treatment 6 years.
The following is a comparison of her before and after treatment:
|
|
|
|
|
|
Changes of bone metastases in patients with recurrent breast cancer after 4 months of ACTL treatment
Now she can no longer see any traces of cancer patients, she is no different from ordinary people, and even better than normal people's mental state. Breast cancer did not make her feel bad, but let her start a new life. In the past few years of her struggle with breast cancer, she told me: "She has gained far more than she has lost."
After listening to my friend, Li Wei (pseudonym) said: "This makes her more confident. She can definitely defeat the devil of breast cancer and enjoy her daily life." Here I would also like to say to all women that breast health needs to be carefully taken care of. It is a must for every woman to develop awareness of breast self-prevention inspection. If you are tired, don't hold on, rest and sleep! The sky fell and let the tall one bear it. If you are bored, make yourself happy! Let the trouble time shorten and then shorten. If you feel wronged, cry bitterly and vent all negative energy! Then wash your face and smile at yourself in the mirror and tell the person in the mirror that you are the most important thing.

"Born as Summer Flowers" is a famous poem by the poet Tagore. He told us: Life is beautiful, and you must live as gorgeous as summer flowers, and you must work hard to make yourself bloom; life will inevitably have all kinds of unsatisfactory, even sadness. , We can still deal with it indifferently, accepting this ending as quietly as autumn leaves.
"Cancer", these two words brought me into a new and extremely special field, and made many new and extremely special friends. They were all dipped in their own tears and blood, and continued to write about their sinister and bumpy journeys, and the lives of fighting with death. These vivid and profound stories touched me deeply! Shocked me!

She still has the charm, but she has breast cancer
She, 50 years old, received surgery and chemotherapy after being diagnosed with breast cancer 7 years ago. After repeated examinations, there were no signs of recurrence. Although a lot of economic costs have been paid during this period, life is the most important, and it is worthwhile to exchange for peace. After 6 years, I felt that my body was abnormal, and the results of the hospital examination were like a bolt from the blue: breast cancer recurrence with lymph node and bone metastasis. I had to undergo chemotherapy again and again, but my condition did not get better, and I felt my body was hollowed out, my face was yellow and thin, my hair fell out, and I felt sick and vomiting. At this time, the store that the couple had painstakingly managed was also facing bankruptcy. Misfortune never comes singly!
The double blow made her particularly pessimistic. She met me when she was the most confused. We talked a lot for a long time. From starting her business with her husband to getting married and having children when her children grew up, everything was so difficult. Now that she lives at this age, she thinks it’s okay. No regrets! I don't want to drag the children and husband down, and spend so much money on treatment. I chatted with her for nearly three hours, and later found her husband and children to persuade her, finally dispelling her extreme thoughts.

Smile to life, it will treat you well
With a glimmer of hope, she went to the hospital again for treatment. The doctor enthusiastically received her, and based on her situation, advised her to give up radiotherapy and chemotherapy, and accept ACTL anti-tumor cell immunotherapy, a precise treatment technique. After listening to the doctor's introduction that ACTL treatment has no obvious side effects and does not affect the quality of life, he resolutely signed the informed consent form for treatment.
In response to her situation, the doctor planned an ACTL treatment plan for her, mainly for CEA, BA46 and Her-2/neu three antigen targets. After a course of treatment, that is, 6 ACTL treatments, not only the condition improved significantly, but the whole person looked radiant, the hair was thick, the skin was fair a lot, and he returned to the state before the illness.
I know very well how difficult it is for all of this to come. A beautiful life once again has the joy of rebirth, blooming again, and growing like a summer flower-it is my greatest relief.
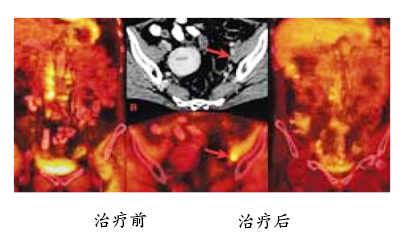
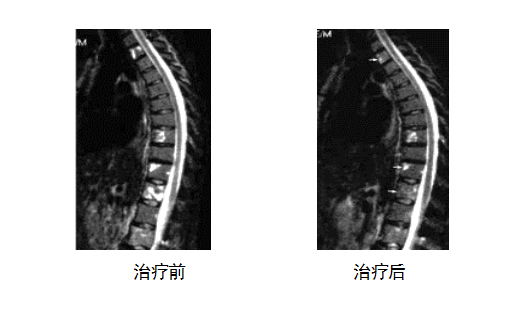
The following left picture shows the situation before treatment, and the metastatic lesions are very obvious after recurrence. The picture on the right shows the results of re-examination after 10 months of ACTL treatment, and the metastatic lesions have disappeared. After more than 5 years of follow-up observation, there was no sign of recurrence.

Prostate cancer is one of the main malignant tumors for middle-aged and elderly men in my country. According to statistics, the incidence of prostate cancer ranks sixth among male malignant tumors in my country, surpassing bladder cancer as the first among male urinary tract tumors, and it is increasing day by day. trend. Early prostate cancer is asymptomatic, and when symptoms appear, it is basically advanced. Some patients missed treatment opportunities because of this. Therefore, it is necessary to carry out publicity and routine screening of prostate cancer knowledge for specific populations, so as to achieve the purpose of early diagnosis and early treatment, and extend the life of patients.
Confirmed to be cancer, collapsed!
In 2009, because of frequent urination and urgency, Mr. Zhang went to the hospital for an examination. The results of the examination showed that Mr. Zhang’s PSA index was higher than normal. Mr. Zhang thought that there was no major problem, so he quickly ignored it.
Six months later, he went to the hospital for an examination again, and the results of this examination left Mr. Zhang dumbfounded. Because he has developed prostate cancer.

A series of terrifying terms "prostate cancer, malignant tumor, advanced stage, metastasis" surrounded Mr. Zhang's ears, causing him to panic. Through the patient's explanation of the doctor, Mr. Zhang underwent surgery and then received anti-hormonal treatment. But three months later, on a review, it was found that his prostate cancer had recurred, and there were bone metastases and lymph nodes.
Don’t give up, there will always be miracles
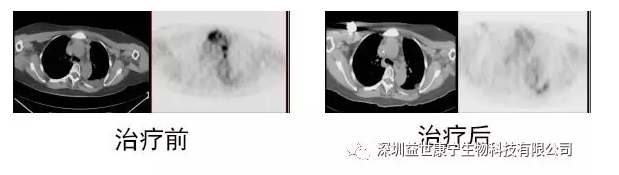
After learning about Mr. Zhang's medical history, imaging data, and pathological evaluation, Mr. Zhang received chemotherapy and radiotherapy in subsequent follow-ups, but there was no obvious effect, and his condition worsened. When Mr. Zhang felt that he could not live long, he accidentally learned about the ACTL treatment technology. After various understandings and consultations, Mr. Zhang received ACTL treatment. After the treatment of Mr. Zhang’s condition, the ACTL immunotherapy with PSA and PSMA antigens was the main treatment. Significant improvement, hormone therapy was effective again, and the PET-CT reexamination results six months after treatment indicated that nearly 98% of the metastatic lesions disappeared. After a return visit, Mr. Zhang has survived for 8 years.
Here are the details of the patient:
|
Patient diagnosis |
: |
Advanced systemic prostate cancer |
|
treatment plan |
: |
Mainly ACTL immunotherapy targeting PSA and PSMA antigens |
|
Treatment result |
: |
The PET-CT review results six months after treatment indicated that nearly 98% of the metastatic lesions disappeared. |
Imaging changes before and after ACTL treatment:

After treatment with hormone therapy and chemotherapy, Mr. Zhang's PSA rebounded three months later and continued chemotherapy, but there was still no improvement, and urinary tract infections and right straddle pain occurred successively. After receiving six ACTL treatments, the PSA dropped and the pain disappeared.
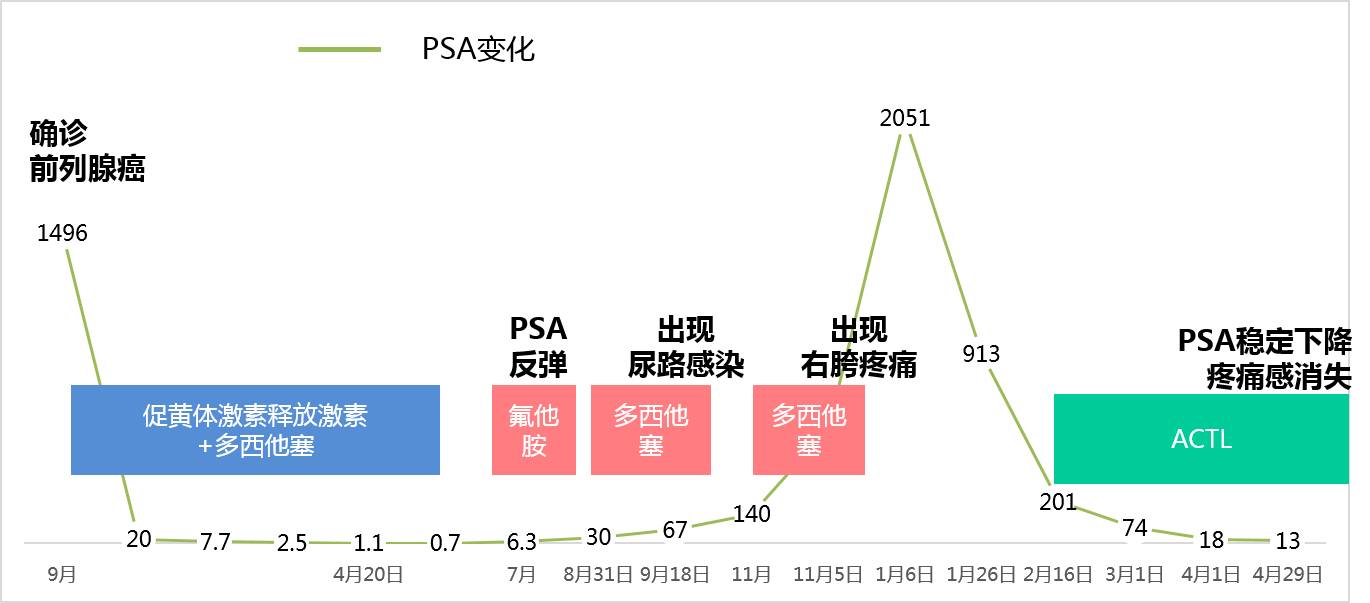
The patient, Mr. Zhang, male, 58 years old, was diagnosed with prostate cancer in September 2014. Check the serum blood tumor marker PSA is 1496, CT scan results showed bone metastasis. In October of the same year, he was given LHRH (Luteinizing Hormone Releasing Hormone) hormone therapy. From January 7th to April 20th, 2015, hormone therapy and chemotherapy (docetaxel) were performed at the same time, and the PSA of the successive tests were (20) (7.7) (2.5) (1.1), and the last test was 0.7 , Is already within the normal range.
In July of the same year, PSA rebounded and rose to 6.3, and received hormone therapy (flutamide), but the effect was not good. From August 31st to September 18th, two consecutive cycles of docetaxel were used, and the PSA was 30 and 67 respectively. Later, due to urinary tract infection, the use of docetaxel was suspended. In November, the PSA value was 140. Docetaxel continued to be used on November 30. In December, the patient reported pain in the right crotch while standing.
At the beginning of 16 years, the patient started ACTL targeted immunotherapy. After treatment, PSA quickly dropped. On April 29, the PSA had dropped to 13. At present, the patient feels good about himself and the pain in his crotch disappears.

In recent years, the incidence of prostate cancer has risen sharply. In European and American countries, the incidence rate is as high as about 20-30%. In North America, prostate cancer has surpassed skin cancer as the most common cancer in men, ranking second in the cause of cancer deaths. The incidence of prostate cancer in my country also reaches or approaches that of European and American countries.
ACTL technology has developed rapidly and has achieved good results in the prevention and treatment of prostate cancer, showing a bright future. The T cells activated by ACTL technology selectively destroy prostate cells that cause cancerous PSA and PSMA abnormal expression, achieving the purpose of treatment.
ACTL treatment has no damage, no toxic side effects, can retain the normal physiological function of the prostate, and overcome the complications and sequelae caused by traditional treatment.
Treatment case
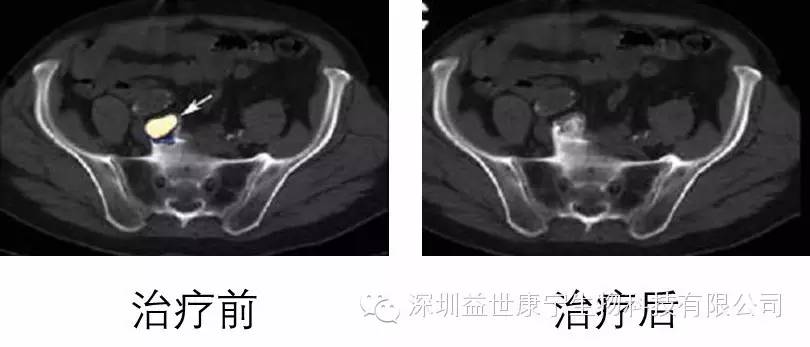
This is a case of advanced prostate cancer. After three months of ACTL treatment, the tumor disappeared in a large area, the patient's mental state was improved, and the quality of life was greatly improved. The target antigens for treatment are PSA and PSMA.
After the occurrence of prostate cancer, the cost of treatment is extremely high. Traditional treatment methods have poor efficacy and high mortality. Therefore, interventional treatment of prostate hyperplasia, that is, preventive treatment, is an effective means to prevent fatal prostate cancer, and has been widely recognized by the academic community.

"I used to regard the check-up report for the diagnosis of cancer as a notice sent by the god of death, telling me that there is not much time. But now, I think this kind of negative and pessimistic idea is not advisable. We must face reality objectively and don't give up. There is a glimmer of hope. Otherwise, I will not be able to receive ACTL targeted cellular immunotherapy, and I will not live today." Mr. Liu said.
Mr. Liu has been suffering from benign prostatic hyperplasia for several years. Although he has increased nocturia and dysuria, due to his busy business, he has not attracted enough attention. It was not until one day that the symptoms became worse and I went to the hospital for an examination and found that it had become cancerous. After receiving surgical castration in a hospital in Hong Kong, Mr. Liu started taking anti-androgen drugs. After being discharged from the hospital, I felt that I was in good health, so I put all my thoughts and energy on my business.

"However, after a year of re-examination, it was found that there was lymph node metastasis. My attending doctor told me that the anti-androgen drugs may have failed. So I started to look for medicine everywhere. After being introduced by a friend, I went to a famous hospital in Guangzhou to receive it. Chemotherapy and radiotherapy. The side effects of chemotherapy are very obvious. I can’t eat food every day and lose a lot of hair. I lose a lot of weight and lose energy all day. After the radiotherapy, I have interstitial pneumonia and a dry cough all day, very uncomfortable. .Actually, I can tolerate all of these, as long as the disease can be cured. But it was counterproductive, not only more extensive lymph node metastasis, but also bone metastasis. The pain of bone metastasis caused me inconvenience. The effect is obvious, and the condition is getting worse. At that time, the doctor in Hong Kong thought that my days were not long and he had nothing to do." Mr. Liu said. For all this sudden situation, Mr. Liu and his family were caught off guard. At that time, Mr. Liu was already discouraged, and he always thought "My life is not long, what should I do with my family, children, and business?"
After learning the news, Mr. Liu's wife did not give up, and once again found the attending doctor in Hong Kong. The attending doctor in Hong Kong told Mr. Liu’s wife: “At present, all treatments in Hong Kong hospitals will not be effective, but I heard that there is a targeted anti-tumor cell immunotherapy called ACTL in Guangdong that has been approved and included in medical insurance in Guangdong. It is recommended Mr. Liu go and try." Mr. Liu was finally persuaded by his wife and tried with the mentality of being a dead horse doctor.
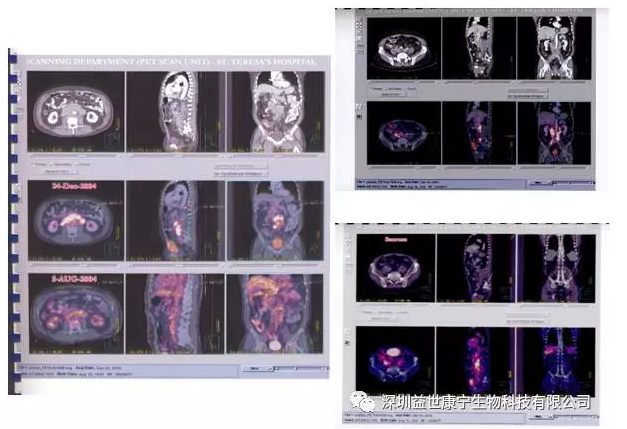
A week later, Mr. Liu finally started to receive ACTL anti-prostate cancer targeted cellular immunotherapy in another well-known hospital in Guangzhou, targeting prostate specific antigen (PSA), prostatic acid phosphatase antigen (PAP) and prostate specific membrane antigen ( PSMA) three targets. The treatment starts every ten days for a total of six times. Later changed to once every fourteen days. The PET-CT review results after 4 months showed that the metastatic lesions basically disappeared (see the figure below). At this time, Mr. Liu felt good about himself, he had no obvious pain before, and he moved freely. Mr. Liu excitedly said: "During several hospitalizations, other patients went in and lie down. But I went in and got out standing. And the business is getting more and more prosperous! This is treated by ACTL. blessing!". For 6 years, Mr. Liu insisted on receiving ACTL technical treatment once every three months.
The following are his imaging changes before and after ACTL treatment:
治疗前
治疗后
A disease is not only a trial to the patient, but also a test of the courage and endurance of the entire family. Perhaps Mr. Liu’s experience is not enough to help you relieve your pain, but I believe that everyone has superb self-healing ability. When you strengthen your belief in self-healing, the door to return to health is also open to you.
Looking back on the days when I was suffering from cancer, it was the company of my family, giving me strength and warmth. It also made me understand that "cancer" is not terrible, but the terrible thing is that I don't have the courage to face it. As long as you have the confidence to face cancer, there is hope for cancer treatment!
The day I learned of cancer, I felt the sky was falling
I am a retired old man. I am 65 years old this year. As people get older, I have various diseases. At first, I just felt uncomfortable in my abdomen, and I always felt faint pain. When I was full, I often felt indigestion. Later, the pain increased, bloody stools also appeared, I often felt fatigued, and my body became thinner. When my wife saw that my appetite had decreased significantly, he asked me where I was unwell? After her inquiries, I told her one to one and went to the hospital with her for an examination.
 After the examination result came out, the whole family kept the secret from me and refused to let me see it. Later, I lost my temper and my wife was tearful, and showed me the test result report. The report showed that the blood carcinoembryonic antigen (CEA) was significantly elevated, and the abdominal CT examination revealed space-occupying lesions of the splenic flexure of the colon. The initial diagnosis was colon cancer. The moment I saw the word "cancer", my mood suddenly exploded in my sky like a bolt from the blue. After reading the inspection report, I didn't say a word. My wife, son and daughter-in-law kept persuading me that it was still early and that there would be nothing wrong with good treatment.
After the examination result came out, the whole family kept the secret from me and refused to let me see it. Later, I lost my temper and my wife was tearful, and showed me the test result report. The report showed that the blood carcinoembryonic antigen (CEA) was significantly elevated, and the abdominal CT examination revealed space-occupying lesions of the splenic flexure of the colon. The initial diagnosis was colon cancer. The moment I saw the word "cancer", my mood suddenly exploded in my sky like a bolt from the blue. After reading the inspection report, I didn't say a word. My wife, son and daughter-in-law kept persuading me that it was still early and that there would be nothing wrong with good treatment.
Family escort gave me the confidence to overcome cancer
In the end, after my wife talked to me, my hope in life was rekindled. She said: "Now that the children are all grown up, they don't want the old father to be frowning all day long. The children now have a lot of pressure in life and they have to worry about the elderly. They are really tired." While my wife was saying these things to me, he recalled the days when I was holding hands warmly. The warm memories of those sufferings made me feel a sense of responsibility as a father and gave me confidence in fighting cancer.
I quickly received surgical resection in the hospital. The operation was successful, and when I woke up, I was surrounded by the warm faces of my relatives. "Dad, okay!" "Grandpa, okay?" The mood at the time was no longer expressible in words. He held back the tears in the corner of his eyes and said, "Okay, everything is fine, there is nothing uncomfortable. ".
Strong affection and love, let the sky of my life shine brightly
After the operation, I received 6 courses of chemotherapy. Although the side effects were obvious, I could still tolerate it. The doctor also thinks that the treatment is ideal. But the good times didn't last long. After 5 months, there was mucus and blood in the stool. He went to the hospital for a review and was told that the cancer had recurred and there were obvious metastatic lesions. So he was discharged again and received 8 courses of chemotherapy. This time, the side effects were particularly serious. Not only were nausea and vomiting, nothing tasted of eating, but also a lot of hair loss. It was a life worse than death. If the treatment is effective, the torture is also worth it. But after the treatment was over, the doctor told me that the effect was not very good, and there was obvious bone marrow suppression, and I could no longer undergo chemotherapy, and asked me to take a good rest. But cancer does not rest!
The son was unwilling to do so, so he consulted and inquired about other treatment methods. When it is learned that ACTL targeted anti-tumor cell immunotherapy may be effective, and that the treatment has small side effects and does not require hospitalization. So I advised me to receive treatment. After immunohistochemical examination, it was determined that the targets of ACTL treatment were carcinoembryonic antigen (CEA), keratin 19 (CK19), prostate-specific membrane antigen (PSMA), and survivin antigen (Survivin). After a period of treatment, the tumor markers dropped to normal and the metabolically increased lesions disappeared. He received a total of 39 treatments with ACTL technology and remained normal after repeated examinations. With the encouragement and support of my wife and children, I insisted on correct and effective treatment, which gave me a chance to be reborn.
The past five years have made me feel the meaning of life, and I live a warmer and more exciting life!
The following is a comparison chart during my treatment with ACTL technology:
|
Determination of CEA and CA19-9 antigen before ACTL treatment |
Determination of CEA and CA19-9 antigen after ACTL treatment |
||||
|
日期 |
CEA |
CA19-9 |
date |
CEA |
CA19-9 |
|
2013.5.7 |
3.21 |
19.61 |
2013.9.11 |
2.40 |
11.51 |
|
2013.6.6 |
9.68↑ |
14.69 |
2014.2.24 |
2.51 |
16.15 |
|
2013.6.7 |
6.25 |
17.87 |
2014.5.16 |
4.06 |
16.87 |
|
|
|
|
2014.6.24 |
4.51 |
21.69 |
|
|
|
|
2014.8.13 |
7.78 |
19.25 |
|
2014.9.23 |
3.58 |
11.38 |
|||
|
2015.6.18 |
3.64 |
16.69 |
|||

Imaging changes after ACTL treatment

For a patient with advanced colon cancer like me, it really feels like a life of nine deaths if I can live till now. If I didn't stick to my beliefs and tried new treatments, I might no longer be alive.
One day four years ago, my body suddenly experienced abdominal distension and indigestion, and then my bowel habits changed. At first I was too busy at work and didn't care too much. But after the bloody stools appeared, I went to the hospital for examination under the orders and kidnapping of my wife and daughter. The results of the examination made me a little confused-colon cancer!

Surgery and chemotherapy treatment made me miserable
I have suffered countless setbacks and blows in my life, and I have survived. Although everyone talks about cancer, I believe that I am in middle age and have good physical fitness. The ghost gate is still far away from me. But the torture of reality completely lost my confidence. Cancer is a disease that deprives people of dignity. This sentence is not false. After being hospitalized, he received surgical resection soon and then received chemotherapy. During chemotherapy, I lost a lot of hair, nauseated and vomited, and nothing was tasted. His body quickly became thin, his face was sallow, and his body was weak. Such days are really difficult and there is no quality of life. When I was discharged from the hospital, the doctor told me to review regularly, which means that I may relapse. My heart is completely cold: How many times will I have to endure before I can enter the ghost gate!
Changing the treatment method showed that the condition improved.
However, whatever you are afraid of. One day after 8 months, I felt a little discomfort in my waist. I was already a frightened bird, so I went to the hospital for examination. The results of the examination gave me a blow: not only the blood tumor markers were significantly increased, but the carcinoembryonic antigen (CEA) was more than 500, and there were large metastatic lesions. The doctor advised me to perform surgical resection again, but the doctor told me that surgical resection does not guarantee that other metastatic lesions will not appear. If you do not have surgery and only do chemotherapy, it cannot be guaranteed to be effective, and it can also be guaranteed that there will be no other metastatic lesions. At that time, I felt my heart cold for a moment, so what is the effect of this operation? Don't you toss yourself? After this knife, there will still be metastasis or recurrence, so how many knives do it have to be useful? Do chemotherapy? Thinking of the last treatment experience, and torturing myself to death, it's better to die!
So I explained my thoughts to the doctor: "Since I can't guarantee that there will be no recurrence, I don't want to do any more treatment, just let it go!" The doctor immediately criticized my negative attitude, thinking that I was resigned and waiting. The idea of death is extremely improper and irresponsible. My wife and daughter also criticized me and took me to the specialist clinic, hoping to find a better treatment. After listening patiently to my nagging, the experts suggested that I try ACTL anti-colon cancer targeted cellular immunotherapy. I also told me that this treatment has no side effects, does not require hospitalization, and does not affect the quality of life. After listening to the doctor's introduction, I had the idea to try this new treatment. My wife also persuaded me that since there are no toxic side effects and it does not affect your quality of life, why not try it? So, I received ACTL targeted cellular immunotherapy.
For my metastatic colon cancer, I received treatments against carcinoembryonic antigen (CEA), keratin 19 type antigen (CK19, CYFRA21-1), survivin antigen (Survivin), prostate specific membrane antigen (PSMA), and melanoma Antigen (MAGE-A3) ACTL targeted cellular immunotherapy with 5 antigen targets. As my metastatic lesions are relatively large and obvious, the doctor advised me to do it once every ten days, and after 6 treatments, change to twice a month. After 2 months of treatment, the blood tumor markers declined rapidly, and his physical condition improved significantly. This gives me the confidence to continue the treatment. After 5 months of treatment, the metastatic lesions were significantly reduced and the blood tumor markers returned to normal. I am full of energy now, and my appetite is bigger than before I got sick. The insomnia that has troubled me for many years is gone. This year I also went to Tibet by car. My wife and daughter said that I had a second spring.
Now I receive ACTL treatment every 3 months. This year is the fifth year, and I am in good physical condition without any recurrence. Everyone wants me to talk about my experience in fighting cancer. In fact, it’s just one sentence: Don’t give up at any time, build self-confidence, follow the doctor’s reasonable advice, work hard and stick to the treatment.

"When I know that I have cancer, I have two choices in my heart. Either lie down quietly and let the cancer go at the mercy of the cancer demon. Or you can change your life and let it go. My son was only 18 years old at the time and he just went to college. I wanted to watch it. Growing up with my son, I decided to fight," Mr. Zhang, who was diagnosed with colorectal cancer at the age of 48, calmly told his cancer story.
Mr. Zhang is a taxi driver. After the Spring Festival in 2010, he had a history of hemorrhoids. He had blood in the stool as usual, but this time he not only had a lot of volume but also had abdominal pain. The pain in the anus was also worse than usual. He went to the hospital for treatment. The examination revealed that Mr. Zhang had colorectal cancer. Mr. Zhang said: "The first reaction to knowing that you have cancer is to infinitely magnify the fear of the unknown. When you put the word cancer on others, you just feel it terrible. In your own body, you are simply sentenced to death, extremely desperate and cold. The soles of the feet. On the way home, I looked at the list that turned out to be cancer. Although I didn't cry, I knew the tears were rolling in my eyes. I thought my son had just entered college and he still had a lot of tuition to earn. I wanted to see it. From graduation to marriage and childbirth, he made up his mind to actively treat."

In March 2010, Mr. Zhang went to the hospital for surgery and then received chemotherapy. After the treatment, Mr. Zhang recovered very quickly. At this time, he felt at ease. Due to busy work and lack of knowledge about cancer, the body did not show any symptoms, so I often did not go for review.
In May 2012, Mr. Zhang felt unwell. Going to the hospital for an examination again, the doctor said that the cancer had recurred and had metastases to the abdominal cavity. "My family is more nervous than me when I hear of recurrence, but I am no longer so scared. In my concept, the god of death is already very close to me. But when my son looked up a lot of information about colorectal cancer, he nailed it up, There are hundreds of pages in total, and the key points are still marked. With the encouragement of my son, I was hospitalized again for chemotherapy." Mr. Zhang said.

"During the period of chemotherapy, repeated blood draws, tests, examinations and infusions, often faced with continuous infusions for several hours, coupled with various physical discomforts, it is easy to make people negative, sometimes lying on the bed Secretly cried." At this point, Mr. Zhang was a little embarrassed and paused for a while. It may be that I remember the scene at that time still vividly, and I have some feelings.
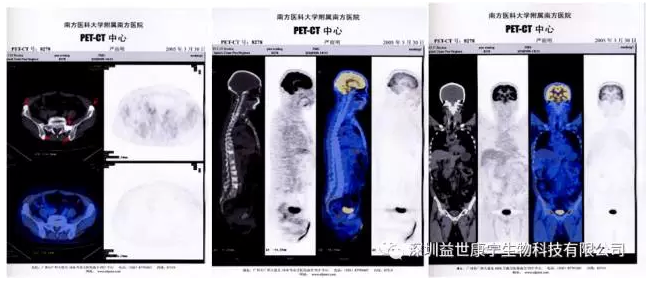
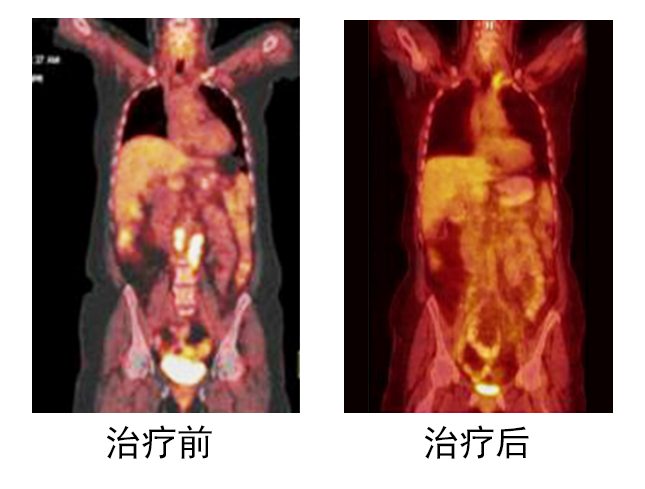
After smiling at me, he continued: "After a period of chemotherapy, my condition has not improved. The doctor advised me to give up chemotherapy and use ACTL targeted anti-tumor cell immune technology. As long as there is a glimmer of hope, I will not give up! After learning about ACTL treatment technology, I resolutely signed an informed consent form for ACTL treatment. The doctor combined my condition and used ACTL for CEA, Her-2/neu and PSMA antigens for 1 year. I received monthly for the first 6 months Two treatments, and then one treatment a month. After the curative effect, the effect is obvious, the pain is gone, and the body feels very comfortable. After the ACTL treatment until now, the body has never felt any discomfort, but feels more energetic I used to drive a taxi for a day, and I always felt very tired and groggy all day. Now I feel that I am more than a decade younger, full of energy, and earn more money from driving in one day than the previous two days." Mr. Zhang said at this time. It can be seen that from his fear of cancer to the difficult course of the treatment process, and the effective treatment, all the ups and downs of this are reflected in his simple face. The following picture is a comparison of Mr. Zhang's condition after 7 months of treatment with ACTL, and the change process of the obvious disappearance of abdominal metastases can be clearly seen:
"As for the future, I don't want to think about it. When I'm alive, I must cherish it and do what I want to do," Mr. Zhang said at last. In fact, many things are precious only when we are about to lose. This is true of life, so is affection, and so is family affection. We always look forward to old days and days, but we don’t know how to manage and cherish them. It’s too late when we truly understand. From this point of view, contentment is better. What perfection is pursued in life? It is best to be able to live happily every day.