Adeno-associated virus (AAV) belongs to the Parvoviridae family. Members of this family are small, non-enveloped, and icosahedral viruses. The diameter of the virus particle is between 20 and 26 nm, and it contains a linear single-stranded DNA genome with a size between 4.7 and 6 kb.
Features of adeno-associated virus
After more than 20 years of extensive research around the world, it has been proved that AAV is a non-pathogenic virus and will not cause any physiological or pathological changes after infecting the human body. The National Institutes of Health (NIH) and the Food and Drug Administration (FDA) have announced for many years that AAV is the safest viral vector for gene therapy. Its characteristics are as follows:
1. Good safety. So far, wild-type AAV has never been found to be pathogenic to humans; recombinant AAV has removed 96% of the wild-type AAV genome, further ensuring safety;
2. Wide host range. Not only can transduce dividing cells, but also transduce quiescent cells;
3. Wild-type AAV can be integrated into the specific position of the q-arm of chromosome 19 of the human genome. Using this feature, it is possible to develop an AAV vector with specific integration function;
4. The genome of AAV-2 is only 4681 nucleotides, which is convenient for operation with conventional recombinant DNA technology;
5. Stable physical properties. Can not be inactivated at 60°C, resistant to chloroform;
6. Recombinant AAV (rAAV) can express foreign genes stably for a long time.
AAV has been widely used in gene therapy and vaccine research worldwide. However, because AAV itself is a replication-defective virus, it is one of the most difficult viral vectors to prepare. There are many obvious defects in application, including the extremely small number of virus receptors on certain cell membranes, insufficient site-specific integration of recombinant AAV vectors, AAV capsid components and transgene products causing host immune responses, and so on. Therefore, it needs the presence of helper virus to produce progeny AAV.
The currently commonly used carrier system is a helper-free packaging system. Its most notable feature is the use of one or several plasmids containing adenovirus or other viral genes with auxiliary functions instead of wild-type adenovirus or other auxiliary viruses, and co-transfecting cells with recombinant AAV viral vectors to obtain infectivity Recombinant adeno associadet virus (rAAV). The biggest benefit of the Helper-Free packaging system is to greatly reduce the production of wild-type adenovirus or other helper viruses, avoid pathogenic virus contamination, and bring great convenience to the post-purification work, thereby making AAV products safe Sexual improvement. However, many Helper-Free packaging systems have some areas for improvement:
1. There is still a certain amount of wild-type adenovirus or other helper virus contamination;
2. It is difficult to completely remove bovine serum albumin and other miscellaneous protein components;
3. The titer of the rAAV virus produced is not high and requires complicated purification and concentration before it can be used in clinical practice;
4. The prepared rAAV has low activity.
Application of AAV in ACTL Technology
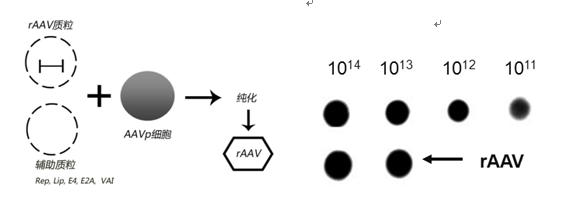
Aiming at the above shortcomings of preparing or producing rAAV, Shenzhen Yishi Kangning Biotechnology Co., Ltd. has greatly improved the production process of rAAV, and basically overcomes these shortcomings. The characteristics of the prepared rAAV products: 1. High titer (see figure); 2. High activity (see figure); 3. No bovine serum albumin or other contaminant proteins; 4. No wild-type adenovirus contamination.
Schematic diagram of virus production. Results of virus titer determination
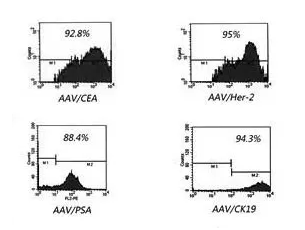
Efficient DC infection rate (high virus activity)
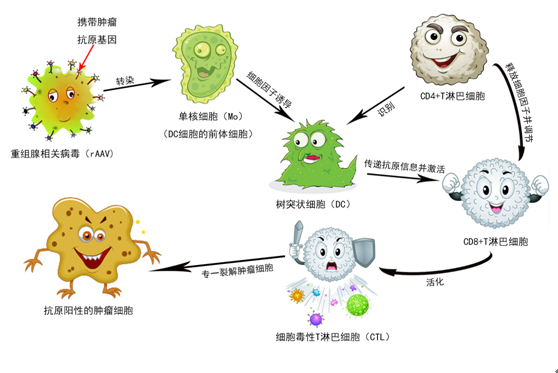
At present, ACTL targeted cellular immunotherapy technology (ACTL technology for short) with independent intellectual property rights in China. The gene vector used in this technology is AAV, which is transformed into rAAV carrying specific tumor-related epitope genes through gene recombination technology , Infects patients’ peripheral blood mononuclear cells, induced by cytokines, the monocytes are transformed into dendritic cells (Denritic cells, DC) with powerful antigen presenting function. The obtained DC can stimulate the production of cytotoxic T lymphocytes (Cytotoxic T ilmphocytes, CTL), which are effective in killing tumor cells, and the generated CTL has tumor antigen specificity, that is, targeting. CTL stimulated by rAAV-infected DCs only have a killing effect on tumor cells that are positive for one or several tumor-associated antigens, and have no effect on antigen-negative cells.
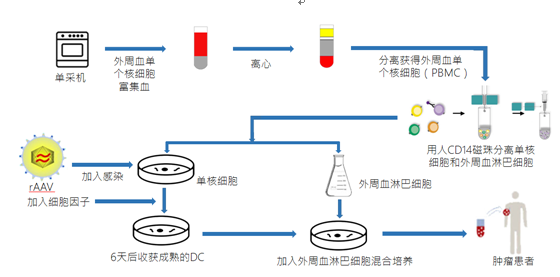
ACTL treatment process:
Physical examination: submit the relevant physical examination report to the attending doctor for evaluation. Those who meet the treatment standards shall sign the patient’s informed consent;
Screening: Perform immunohistochemical detection to screen tumor antigens;
Blood collection: 80-150ml of peripheral blood is drawn from the patient, and the peripheral blood mononuclear cell (PBMC) is isolated;
Cell culture: Induced and cultured in the laboratory for 12-18 days: After culture, PBMC are separated into lymphocytes and monocytes, rAAV virus infects monocytes, and cytokine induces DC production. The mature DC and lymphocytes are mixed culture , Is the induction of lymphocytes into antigen-specific CTLs that kill antigen-positive tumor cells;
Reinfusion: Generally, reinfusion is performed about 14 days after blood collection, and the activated CTL is injected intravenously back to the patient, which is completed within 30 minutes.