Bladder cancer refers to a malignant tumor that occurs on the bladder mucosa. It is the most common malignant tumor of the urinary system and one of the ten most common tumors throughout the body. It accounts for the first place in the incidence of genitourinary tumors in my country, and its incidence is second only to prostate cancer in the West, ranking second. In 2012, the incidence of bladder cancer in the national tumor registration areas was 6.61 per 100,000, ranking 9th in the incidence of malignant tumors. Bladder cancer can occur at any age, even in children. The incidence rate increases with age, with a high incidence age of 50 to 70 years. The incidence of bladder cancer in men is 3 to 4 times that of women.
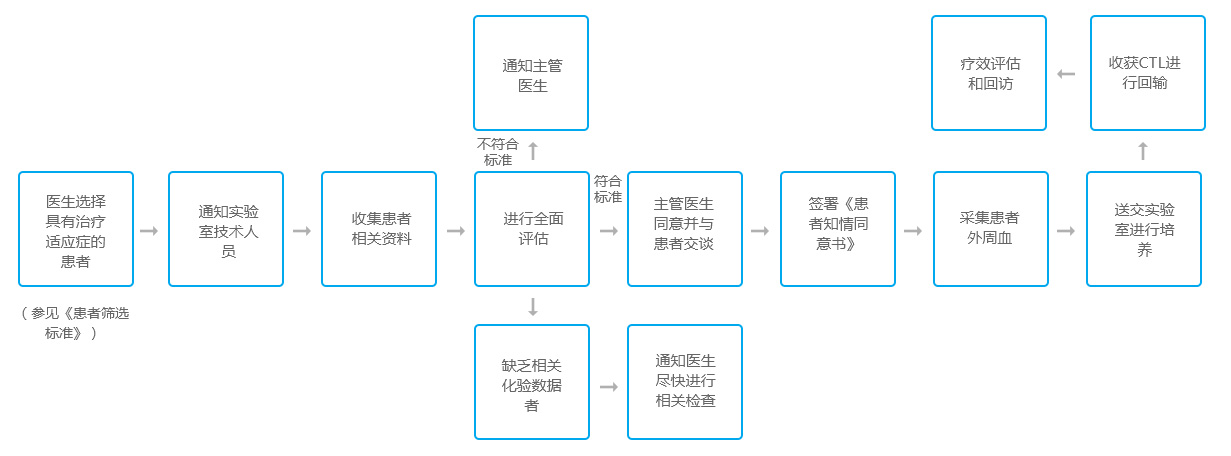
After determining the indications for ACTL treatment of patients in accordance with the "Screening Criteria for Cancer Patients", the specific ACTL treatment plan should be determined according to the specific conditions of the patient, such as the patient's condition, receiving radiotherapy and chemotherapy, and the following principles should be mastered:
1. The patient should have blood routine, liver and kidney function, relevant serum tumor markers (tumor antigen or tumor-related antigen) laboratory test and imaging examination results.
2. During ACTL treatment, in addition to treatments that damage immune function or bone marrow suppression, patients can receive other anti-tumor treatments.
3. The number of antigen-specific cytotoxic T Iymphocytes (CTL) of patients who are reinfused in each treatment should be ≥1*108
4. For patients with allergies or elderly and infirm, when CTL is reinfused, a small dose of dexamethasone can be considered.
5. Clinical therapeutic grade interleukin 2 (IL-2): At the same time as CTL is reinfused, intravenous infusion of IL-2 is recommended to adjust the patient's immune function, prolong the activity of CTL in the body, and improve the efficiency of killing tumor cells. For the first reinfusion, the recommended dose is 500,000 units to observe the patient's tolerance to IL-2. If the patient has no obvious adverse reactions or allergic reactions, from the second ACTL treatment, 1 to 2 million units of IL-2 will be given each time CTL is infused. It is not recommended to use my country's SFDA approved for clinical treatment
After determining the indications for ACTL treatment of patients in accordance with the "Screening Criteria for Cancer Patients", the specific ACTL treatment plan should be determined according to the specific conditions of the patient, such as the patient's condition, receiving radiotherapy and chemotherapy, and the following principles should be mastered:
1. The patient should have blood routine, liver and kidney function, relevant serum tumor markers (tumor antigen or tumor-related antigen) laboratory test and imaging examination results.
2. During ACTL treatment, in addition to treatments that damage immune function or bone marrow suppression, patients can receive other anti-tumor treatments.
3. The number of antigen-specific cytotoxic T Iymphocytes (CTL) of patients who are reinfused in each treatment should be ≥1*108
4. For patients with allergies or elderly and infirm, when CTL is reinfused, a small dose of dexamethasone can be considered.
5. Clinical therapeutic grade interleukin 2 (IL-2): At the same time as CTL is reinfused, intravenous infusion of IL-2 is recommended to adjust the patient's immune function, prolong the activity of CTL in the body, and improve the efficiency of killing tumor cells. For the first reinfusion, the recommended dose is 500,000 units to observe the patient's tolerance to IL-2. If the patient has no obvious adverse reactions or allergic reactions, from the second ACTL treatment, 1 to 2 million units of IL-2 will be given each time CTL is infused. It is not recommended to use my country's SFDA approved for clinical treatment
After determining the indications for ACTL treatment of patients in accordance with the "Screening Criteria for Cancer Patients", the specific ACTL treatment plan should be determined according to the specific conditions of the patient, such as the patient's condition, receiving radiotherapy and chemotherapy, and the following principles should be mastered:
1. The patient should have blood routine, liver and kidney function, relevant serum tumor markers (tumor antigen or tumor-related antigen) laboratory test and imaging examination results.
2. During ACTL treatment, in addition to treatments that damage immune function or bone marrow suppression, patients can receive other anti-tumor treatments.
3. The number of antigen-specific cytotoxic T Iymphocytes (CTL) of patients who are reinfused in each treatment should be ≥1*108
4. For patients with allergies or elderly and infirm, when CTL is reinfused, a small dose of dexamethasone can be considered.
5. Clinical therapeutic grade interleukin 2 (IL-2): At the same time as CTL is reinfused, intravenous infusion of IL-2 is recommended to adjust the patient's immune function, prolong the activity of CTL in the body, and improve the efficiency of killing tumor cells. For the first reinfusion, the recommended dose is 500,000 units to observe the patient's tolerance to IL-2. If the patient has no obvious adverse reactions or allergic reactions, from the second ACTL treatment, 1 to 2 million units of IL-2 will be given each time CTL is infused. It is not recommended to use my country's SFDA approved for clinical treatment
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are ready to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are ready to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are ready to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are ready to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are ready to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
1. Patients who are receiving radiotherapy and chemotherapy are not recommended to receive ACTL treatment at the same time;
2. For patients who are going to receive radiotherapy or chemotherapy, consider the following ACTL treatment plan, but pay attention to the obvious decline in the number of white blood cells or bone marrow suppression, and stop ACTL treatment;
