
11
05
The most common targeted therapy for non-small cell lung adenocarcinoma (NSCLC) is epidermal cell growth factor receptor inhibitor (EGFR). For example: Gefitinib and so on. However, some patients with good results will develop resistance to small-molecule targeted drug therapy after a period of time.
With the development of medical technology, biological therapy has become one of the main methods of cancer treatment. Our clinical research results show that it is a biological therapy. ACTL anti-tumor targeted cellular immunotherapy (ACTL) with independent intellectual property rights not only has a good effect on the treatment of non-small cell lung adenocarcinoma, but also may reverse drug resistance. Sex.
A clinical study on the effectiveness of ACTL therapy in 21 patients with non-small cell lung adenocarcinoma (NSCLC) in reversing or prolonging resistance to gefitinib
Inclusion criteria of patients: had received surgical treatment, met the indications for gefitinib treatment, and immunohistochemical detection of tumor tissue carcinoembryonic antigen (CEA), keratin 19 antigen (CK19), melanoma-associated antigen A3 (MAGE- A3) Survivin antigen (Survivin), prostate specific membrane antigen (PSMA) and mucin antigen (MUC-1) are abnormal, and at least two of them are positive. Tumor tissue HLA-I antigen is positive, and peripheral blood leukocytes are ≥ 3.0×109/L. The target antigen for ACTL treatment: the above-mentioned antigens are positive patients.
Treatment plan: 66 patients with non-small cell lung adenocarcinoma were randomly divided into experimental group and control group. During the study period, the serum CEA, CK19 and imaging changes of NSCLC patients receiving treatment were regularly observed for a maximum period of 14 months.
Test group: 31 cases. The course of ACTL treatment is 6 months, a total of 12 treatments, one treatment every 14 days. Each return is ≥2×108 CTL, and the return is completed within 30 minutes. During the treatment, gefitinib was routinely taken at the same time, and no other antitumor drugs were given. After ACTL treatment, gefitinib alone was continued.
Control group: 35 cases. Gefitinib alone, no other anti-tumor drugs.
Effective observation indicators for treatment: 1) changes in serum tumor markers: a decrease of >50%; or/and 2) changes in imaging: tumor burden decreased or disappeared or remained unchanged.
The treatment research results of the experimental group:
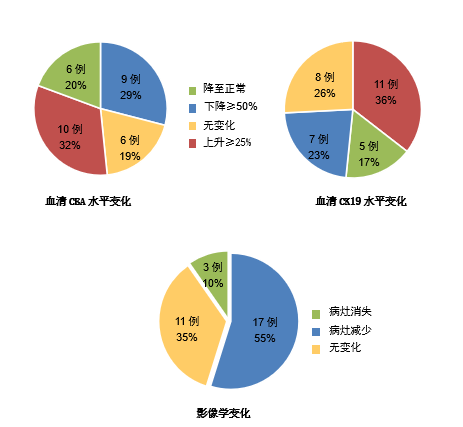
After 6 months of treatment with ACTL+gefitinib among 31 NSCLC patients, 21 of them were judged to be effective in combination therapy, and after continuing to use gefitinib alone for 8 months, the results were compared with the treatment results of the control group. The following table is a summary of the efficacy of the experimental group and the control group.
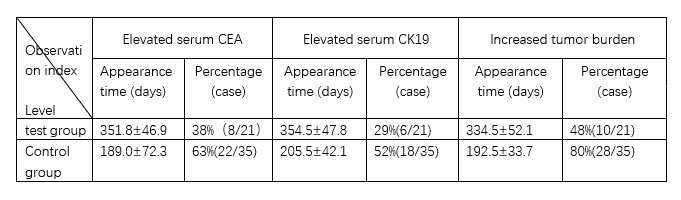
The results of the study showed that from the observation of serum tumor markers and imaging studies, there were significant differences between the test group and the control group (p<0.05). It suggests that ACTL treatment may block or delay the occurrence of gefitinib resistance.



