
03
02
As the living standards of our people are improving, the incidence of prostate cancer is also on the rise. At present, cancer immunotherapy has become the fourth mode of treatment after surgery, chemotherapy and radiotherapy. Years of clinical treatment practice proved that ACTL targeted cellular immunotherapy products and derived technologies have achieved satisfactory preliminary results in the treatment of prostate cancer. In addition, the cytotoxic T lymphocytes (CTL), which are prepared by technology with independent intellectual property rights in my country, have the characteristics of high efficiency, targeting and slight side effects, and the characteristics of precise treatment are very prominent.
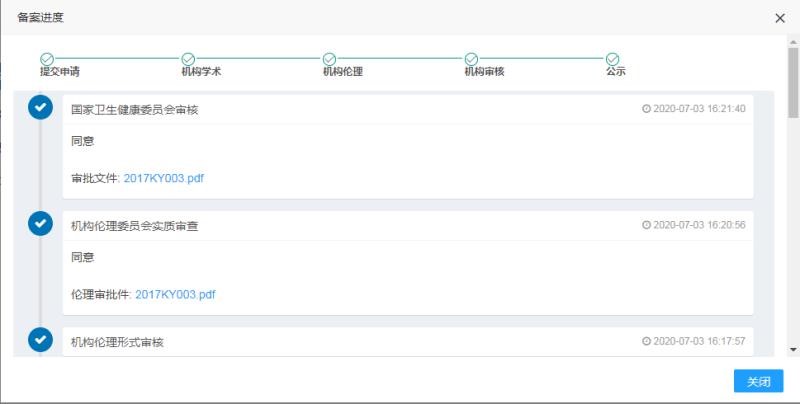
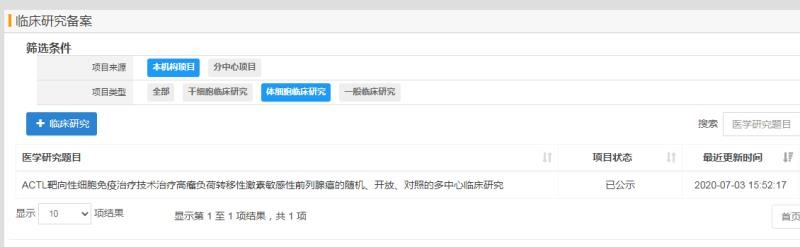
In July 2020, the "ACTL Targeted Cellular Immunotherapy Technology for the Treatment of High Tumor Burden Metastatic Sensitive Prostate Cancer Randomized, Open, Controlled Multicenter Clinical Study" project carried out by Zhejiang Provincial People's Hospital has successfully passed the National Health Commission Approval and publicity.
This clinical trial will conduct the clinical efficacy and safety study of multi-target ACTL targeted cellular immunotherapy for prostate cancer. The target antigens are prostate specific antigen (PSA), prostate specific membrane antigen (PSMA) and prostate Acid Phosphatase (PAP). The aim is to study the application of this treatment technology on the basis of endocrine therapy to prolong the progression-free survival (PFS) of patients, and to evaluate the role of ACTL treatment technology in the personalized targeted therapy of prostate cancer, and to develop a standard for precise treatment. At the same time, combined with clinical practice, the interaction between tumor cells and host immune cells in the development of prostate cancer is studied at the molecular, cellular and overall levels, revealing the pathophysiological process and mechanism of host immune system suppression by tumors, and exploring the importance of immune suppression in the body The activation pathways and strategies provide new theories and new methods for the treatment of prostate cancer, and can also provide clinical evidence-based basis for the targeted cellular immunotherapy of prostate cancer in the future.
By using specific immune cells against the above three target antigens prepared according to the individual conditions of the subject for multi-target targeted therapy, it will provide more methods and new technologies for the cellular immunotherapy of prostate cancer, as well as clinical applications Standard formulation and update of technical standards provide references, enabling my country to rank among the international leaders in the field of precision treatment of prostate cancer.
ISKCON will continue to work with well-known domestic and foreign medical institutions, scientific research institutes, and biotechnology companies to continuously promote the clinical research and medical transformation of ACTL technology to benefit the majority of cancer patients.



