
02
02
A well-known international authoritative journal in the field of clinical lung cancer diagnosis and treatment, "Clinical Lung Cancer", published a report about ACTL targeted cellular immunotherapy (ACTL) for the treatment of non-small cell lung adenocarcinoma (NSCLC) brain metastases. (That is, the adoptive T-cell transfer (ATCT) Therapy mentioned in the article). Many scientists and doctors, including Massachusetts General Hospital and MD Anderson Cancer Research Center, sent emails and phone calls for consultation, suggesting that further clinical research on ACTL treatment for lung cancer brain metastasis should be carried out as soon as possible, and expressed cooperative research to jointly overcome this Willingness for medical problems.
The brain is a common distant metastasis site for NSCLC. About 40% of NSCLC patients will have brain metastases, and the overall survival of these patients is only 3 to 6 months. Most drugs are ineffective due to difficulty in passing through the blood-brain barrier. Therefore, there are very few effective treatments for brain metastases, which is one of the biggest problems in clinical treatment.
ACTL treatment experts observed and evaluated the effects of ACTL treatment in 3 NSCLC patients with brain metastases. Three patients who had received surgical treatment received chemotherapy after they developed resistance to a small molecule targeted drug (gefitinib). However, due to obvious brain metastases, the treatment was terminated and ACTL was accepted. The targets (target antigens) of ACTL treatment are carcinoembryonic antigen (CEA), keratin 19 (CK19) and prostate specific membrane antigen (PSMA). Each patient received ACTL treatment once every two weeks, and the treatment lasted more than 3 months. No other treatments were received during the treatment.
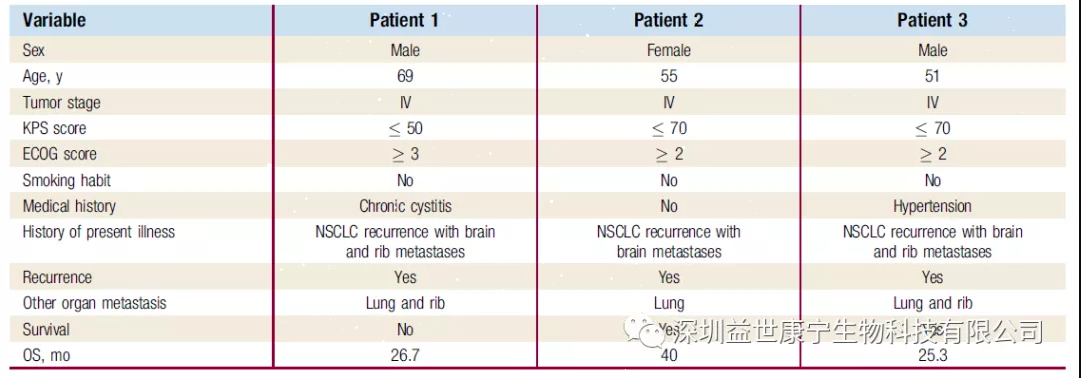
Details of NSCLC patients
After ACTL treatment, the efficacy of ACTL was evaluated. The serum CEA levels of 3 patients returned to normal. The overall response to treatment was evaluated as stable (SD) and partial response (PR). The most important thing is that the results of MRI showed that the brain metastases disappeared. Except for one patient who died due to multiple organ failure, whose overall survival (OS) was 26.7 months, the other two patients were still alive after the end of the study, with OS reaching 25.3 months and 40 months, respectively.
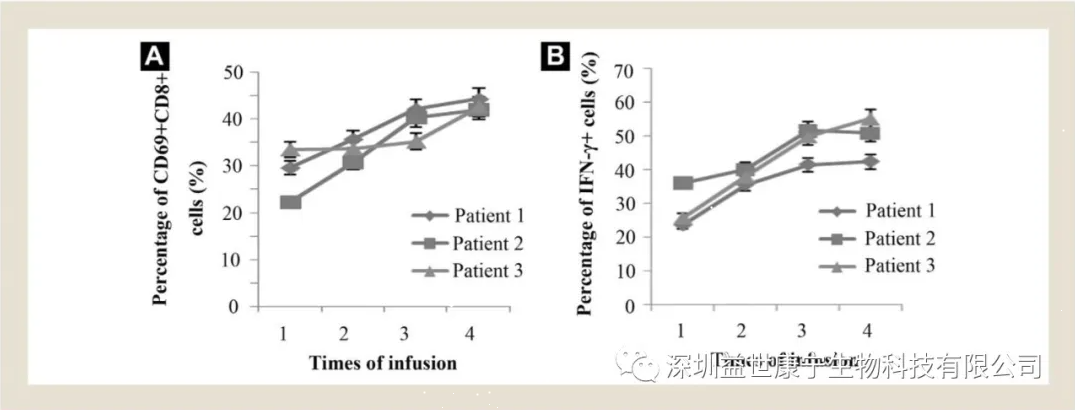
Changes of CD69+/CD8+ T lymphocytes and gamma interferon (IFN-γ) before and after treatment
During ACTL treatment, it was found that the number of CD69+/CD8+ T lymphocytes in the patient increased and the level of interferon-gamma (IFN-γ) expression in the cells increased, indicating that the patient’s cellular immune function was enhanced, and the effector cells, namely cytotoxic T lymphocyte The ability of cells (CTL) to kill cancer cells is gradually increasing. This once again proves that ACTL not only plays the main therapeutic effect of targeted killing of cancer cells, but also adjusts the patient's overall immune function, especially the improvement of cellular immune function. This is extremely beneficial to improve the overall cancer treatment effect.
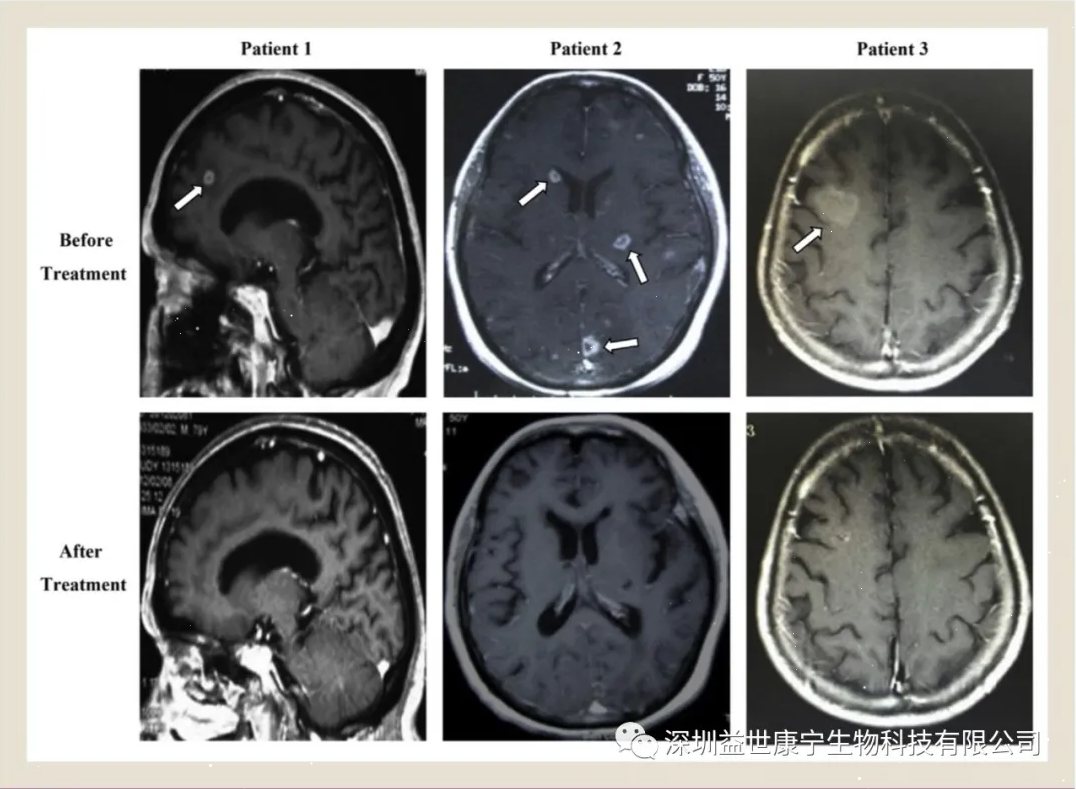
1 month before and after treatment, the results of brain magnetic resonance imaging scans, the arrows indicate brain metastases
Experts believe that ACTL may be effective in treating brain metastases from lung cancer and is worthy of in-depth study. The patient not only got relief from ACTL treatment, but also had no obvious serious side effects. It is expected to achieve faster clinical applications and benefit more patients.
Reference materials:
Clinical Lung Cancer, Vol. 21, No. 4, e270-3 ©2020 Elsevier Inc.



