In 2009, internationally recommended and formulated a new standard for the efficacy of tumor immunotherapy—Immune-Related Response Criteria (irRC) to make up for the fact that RECIST or the improved WHO standard is not fully applicable to anti-tumor cell immunotherapy defect.
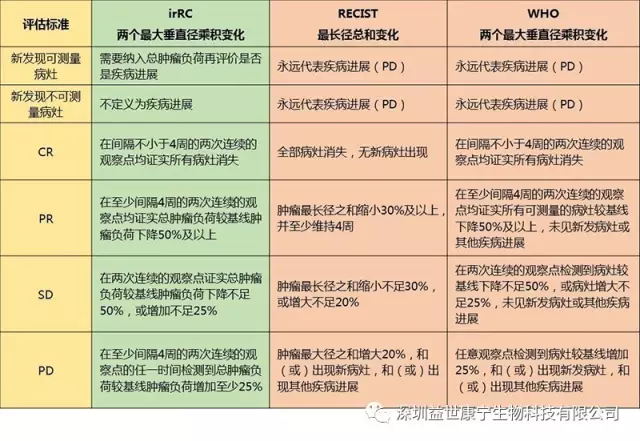
Comparison of irRC standard with RECIST and WHO standard
Shortcomings of RECIST and WHO standards
Imaging data is used as the only criterion for the evaluation of curative effect, and the overall therapeutic effect is judged by the local curative effect.
Only the tumor shrinkage lasted for more than 4 weeks to evaluate, which reflects the recent curative effect.
A single objective standard is used to reflect complex human diseases, ignoring people's subjective feelings and survival time.
The innovation of irRC standard
Measurable new lesions were included in the total tumor burden and compared with the baseline tumor burden.
The initial evaluation is irPD. If the condition does not deteriorate rapidly, treatment and a second evaluation are still needed, because it is likely to start to shrink within 4 weeks after the determination of irPD. Only two consecutive evaluations have an increase in tumor burden, and it is greater than 25% are recognized as irPD.
For those patients with irSD whose tumor burden decreases slowly, although more than 25% but less than 50%, they are considered to be clinically beneficial.
In the practice of immunotherapy, the discovery of new lesions in tumor patients does not necessarily indicate that the treatment is ineffective. A considerable proportion of patients continue to receive treatment and suffer from SD, PR or even CR. Most immunotherapy works slowly, and the appearance of new lesions or a slight increase in tumor burden in a short period of time is not necessarily a deterioration. More importantly, immune cells attack tumor cells, causing massive necrosis of tumor cells, which leads to tumor tissue edema, which usually increases tumor volume, which can easily lead to misjudgment of disease progression.
In addition, in 2014, the American Society of Clinical Oncology (ASCO) cited breast cancer-related serum tumor markers (tumor-associated antigens) as an example that is not necessarily a manifestation of ineffective treatment. It is recommended that tumor patients experience elevated serum tumor markers. , Can not simply think that the disease has worsened.
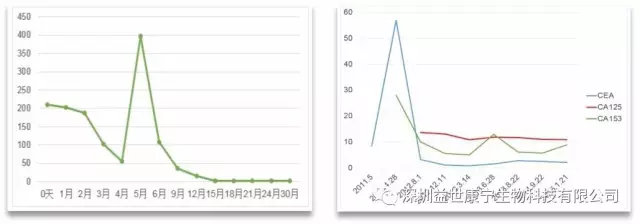
In the process of ACTL anti-tumor targeted cellular immunotherapy, the serum tumor markers of tumor patients with effective treatment often increase, and they usually last for 45-60 days, and then appear to decrease significantly, usually accompanied by a decrease in tumor burden. It is believed that its possible mechanism is that tumor cells are killed (lysed), resulting in a large amount of tumor cell lysates including tumor-associated antigens and tumor antigen proteins being released into the blood circulation. However, some scholars believe that tumor cells reflexively increase the secretion of antigen proteins in order to maintain the stability of the microenvironment. This is different from the clinical manifestations of patients whose disease has deteriorated. Such patients often exhibit no aggravation or no symptoms.
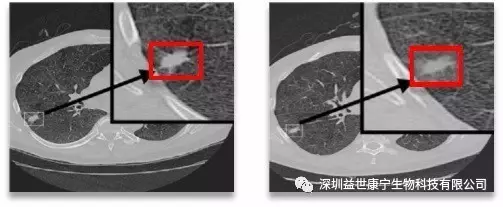
Treatment case
The figure below shows a typical case of using irRC criteria to evaluate the efficacy of ACTL. After the patient received treatment, the tumor lesions did not shrink, but the density was significantly reduced, indicating that a large number of tumor cells were necrotic and the effect was obvious.
The following two figures show the changes in serum tumor markers of patients who received ACTL treatment according to the irRC standard and the curative effect reached CR. During the treatment, serum tumor markers increased temporarily, and then continued to drop to normal levels, and the tumor burden disappeared.