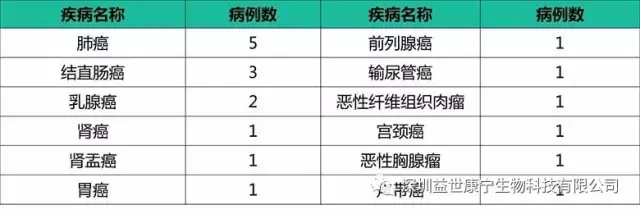
Recently, we reviewed the investigational treatment of a group of patients. All 19 patients started ACTL treatment after the previous treatment failed. These include common solid tumors such as lung cancer and colorectal cancer, as well as rare cancers such as thymoma.
These patients are all in stage IV tumors (with clear distant metastasis and lymph node metastasis outside the primary lesion), and they are all patients who have failed previous treatment.
safety
During the treatment, the safety was good and no serious side effects occurred. One of the patients developed fever symptoms and then relieved spontaneously; 2 patients developed a state of excitement during the first 3 cell infusions, and there were no other obvious side effects; other patients No side effects occurred. It shows that ACTL treatment is a safe treatment method.
Effectiveness
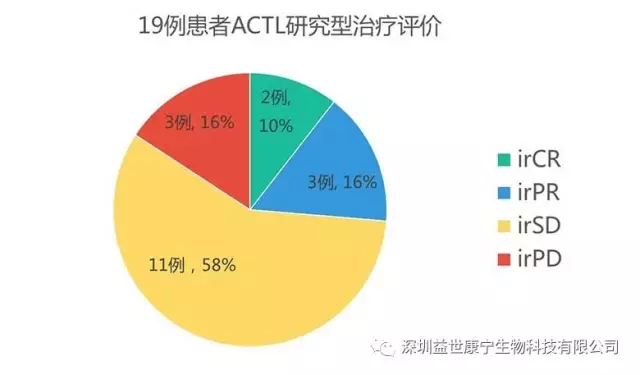
irRC is the evaluation standard for the effectiveness of anti-tumor immunotherapy recommended by ASCO. Among them, the effective treatments are:
irCR:: Complete Remission, that is, complete remission
irPR:: Partial Remission, that is, partial remission
irSD: Stable Disease, that is, stable disease
Those who judged treatment failure are:
irPD: Progressive Disease, that is, disease progression
Among the 19 patients in the statistics, 26% of the comprehensive evaluation indicators were remission, 58% were stable, and only 16% were disease progression.
to sum up
ACTL is a safe and effective cancer treatment technology that can be used for the treatment of a variety of cancers. It has good effects and low side effects. It can effectively kill cancer cells and has an obvious effect in stabilizing the condition and preventing the spread of tumors. On the basis of protecting the patient's autoimmune function, it can significantly improve the patient's quality of life.